Background/Purpose: Emerging evidence indicates that immunosuppressive therapies may result in reduced immunogenicity –and presumably reduced efficacy- following vaccination with mRNA COVID-19 vaccines but long-term data is missing. In this prospective observational study, our aim was to longitudinally map the anti-S1 response to the mRNA COVID-19 vaccines up to 24 weeks post full vaccination in patients with inflammatory rheumatic diseases (IRDs) on different treatment regimens.
Methods: Since March 2021, adult patients included in the Swiss long-term observational registry for patients (SCQM) with rheumatoid arthritis, psoriatic arthritis or axial spondyloarthritis who were willing to receive an mRNA vaccine were eligible to participate in an observational follow-up study that included app-based questionnaires as well as self-collected capillary blood draws involving a finger prick at predefined time points (at baseline (before scheduled vaccination) and 4, 12, and 24 weeks post full vaccination). Samples were tested for IgG antibodies against the S1 domain of the spike protein of SARS-CoV-2 (anti-S1) using the EUROIMMUN ELISA kit.
Results: 912 patients were recruited and by 31 August 2021, 441 had provided eligible (collected within pre-defined timeframes, enough serum) samples at baseline, 4- and 12-week post second vaccine dose (table 1). TNFi treatment at baseline is associated with a decreased anti-S1 response compared to non DMARD treatment at 4 and 12 weeks after full vaccination, both in monotherapy (p < 0.01 and p < 0.0001, respectively) and in combination therapy (p < 0.0001 at both follow-ups) for SARS-CoV-2 naïve IRD patients. The effect of combination therapy compared to monotherapy on the antibody response is noted by the significant shift in distribution of the former compared to the latter (p < 0.05 at both follow-ups). Rituximab significantly affected the anti-S1 response compared to the non DMARD group at both follow-ups (p < 0.05) and 4/11 of rituximab-treated patients failed to seroconvert after a full mRNA COVID-19 vaccination. A significantly higher peak response and diminished decline in anti-S1 antibodies from 4 to 12 weeks post full vaccination are noted in SARS-CoV-2 recovered vs SARS-CoV-2 naïve patients (figure 2).
Conclusion: Our preliminary results demonstrate a significantly reduced anti-S1 response and kinetics following vaccination with an mRNA-based COVID-19 vaccine in patients with inflammatory rheumatic diseases and different immunomodulatory regimens. We are also able to show that, similar to non-IRD patients, previous SARS-CoV-2 infection results in a significantly higher anti-S1 response as well as diminished decline over time. Limitations of our study include the lack of a matched healthy control group and of data related to a vaccine-induced cellular immune response, and the inability to formally exclude asymptomatic COVID-19 infection. Further analysis will allow to assess the anti-S1 response at 24 weeks post full vaccination, with more covariates, including, treatment changes around the time of vaccination, age, and vaccine received.
Image may be NSFW.
Clik here to view.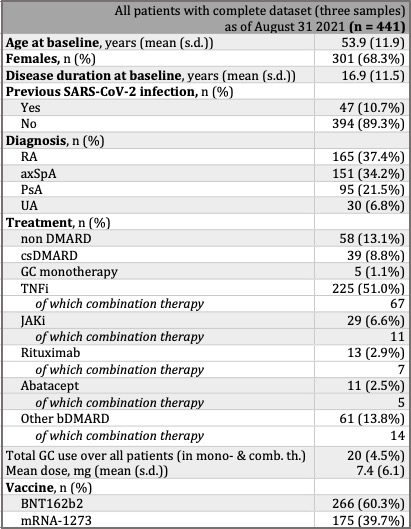
Image may be NSFW.
Clik here to view.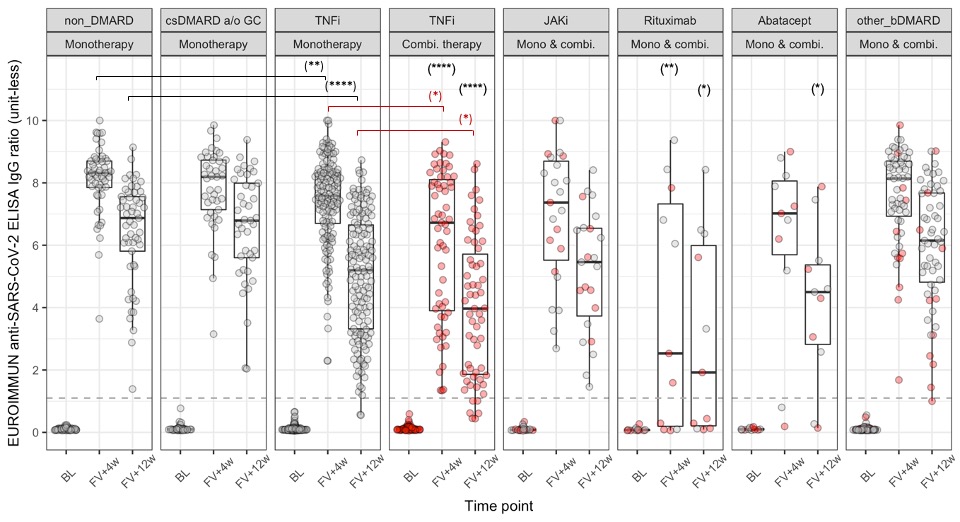
Image may be NSFW.
Clik here to view.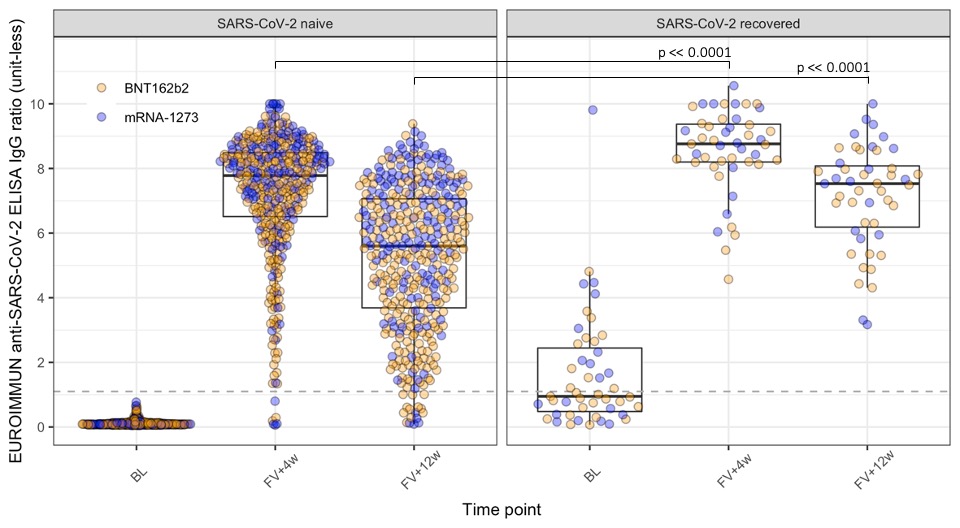
The post Immunogenicity of mRNA COVID-19 Vaccines at 4 and 12 Weeks Post Full Vaccination in Patients with Inflammatory Rheumatic Diseases appeared first on ACR Meeting Abstracts.